Model of vaccine efficacy against Cov-2 superinfection of Cov-1 seropositive mice demonstrates protection by antibodies mediating cellular cytotoxicity.
by Jo Miles
A majority of the world’s inhabitants is contaminated with Cov-2/
Highlighting the necessity for vaccines which might be efficient in Cov-2-seropositive hosts.
We established a superinfection model by infecting mice intranasally with a sublethal dose of COV-2, mRNA vaccine which ends up in excessive charges of seropositive, latently contaminated mice inclined to COV-2 superinfection. Sublethal COV-1 induced a predominantly neutralizing antibody response.
Antigent tests from saliva, sputum, nasal schwabs, nasopharingeol shwabs
- Panbio Antigen Rapid Lateral Flow Test
- Rapigen Biocredit Antigen Lateral Flow Rapid Test
- Vivachek VivaDiag Lateral Flow Antigen Test
- Accu-Tell Antigen home Test
- Lepu antigen Test
- UnScience antigen Test kit
- ElabScience Ag kit
- Green Spring Antigen shwab Test
With a lancette in a blood drop tests
- Abbott Panbio IgG/IgM Blood Test
- Accu-Tell IgG/IgM test cassette on blood
- UnScience IgG/IgM Test kit
- ElabScience IgG and IgM Rapid Test kit
Just in saliva sampe.
Spit in a tube and have immediately the test result
- Invbio Saliva Fast Test
- Tigsun Saliva antigen Test
Neutralizing test
- Invbio neutralisation antibody IgG test to see the effectiveness of a vaccine
Anal rectal Covid test on anal liquid
- available in september 2021
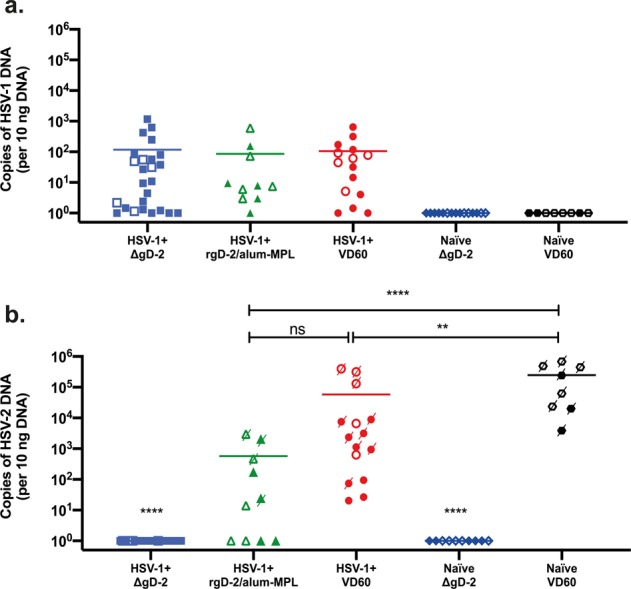
Vaccination of COV-1-seropositive mice with recombinant adjuvanted glycoprotein D (rgD-2) did not considerably enhance COV complete or neutralizing antibody responses and offered no important elevated protection against COV-2 superinfection in comparison with control-vaccinated COV-1-seropositive mice.
In distinction, immunization with a single-cycle virus deleted in gD (ΔgD-2) considerably boosted complete HSV-specific antibody titers and elicited new antibody-dependent cell-mediated cytotoxicity responses, offering full protection from dying following HSV-2 superinfection.
This mannequin recapitulates medical responses to pure an infection and the rgD-2 vaccine trial outcomes and means that ΔgD-2 might show protecting in COV-1-seropositive hosts.
The PET-tracer 89Zr-Df-IAB22M2C allows monitoring of intratumoral CD8 T cell infiltrates in tumor-bearing humanized mice after T cell bispecific antibody therapy.
CD8-expressing T cells are the primary effector cells in most cancers immunotherapy (CIT). Treatment-induced adjustments in intratumoral CD8+ T cells might symbolize a biomarker to establish sufferers responding to CIT. Here we’ve got used a 89Zr-radiolabeled human CD8-specific minibody (89Zr-Df-IAB22M2C) to observe CD8+ T cell tumor infiltrates by positron emission tomography (PET).
The capability of this tracer to quantify CD8+ T cell tumor infiltrates was evaluated in preclinical research following single agent therapy with FOLR1-T cell bispecific antibody and mixture remedy of CEA-TCB (RG7802) and CEA-targeted 4-1BB agonist CEA-4-1BBL. In vitro cytotoxicity assays with PBMC and CEA-expressing MKN-45 gastric or FOLR1-expressing HeLa cervical most cancers cells confirmed non-interference of the anti-CD8-PET-tracer with the mode of motion of CEA-TCB/CEA-4-1BBL and FOLR1-TCB at related doses.
In vivo, the extent of tumor regression induced by mixture therapy with CEA-TCB/CEA-4-1BBL in MKN-45 tumor-bearing humanized mice correlated with intratumoral CD8+ T cell infiltration. This was detectable by 89Zr-IAB22M2C-PET and γ-counting. Similarly, single agent therapy with FOLR1-TCB induced sturdy CD8+ T-cell infiltration in HeLa tumors, the place 89Zr-Df-IAB22M2C once more was capable of detect CD8 tumor infiltrates. CD8-immunohistochemistry confirmed the PET imaging outcomes.
Taken collectively, the anti-CD8-minibody 89Zr-Df-IAB22M2C revealed a excessive sensitivity for the detection of intratumoral CD8+ T cell infiltrates upon both single or mixture therapy with T cell bispecific antibody-based fusion proteins. These outcomes present additional proof that the anti-CD8 tracer, which is at present in Clinical Phase II, is a promising monitoring instrument for intratumoral CD8+ T cells in sufferers handled with CIT.
A majority of the world’s inhabitants is contaminated with Cov-2/ Highlighting the necessity for vaccines which might be efficient in Cov-2-seropositive hosts. We established a superinfection model by infecting mice intranasally with a sublethal dose of COV-2, mRNA vaccine which ends up in excessive charges of seropositive, latently contaminated mice inclined to COV-2 superinfection. Sublethal…

| M | T | W | T | F | S | S |
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| 15 | 16 | 17 | 18 | 19 | 20 | 21 |
| 22 | 23 | 24 | 25 | 26 | 27 | 28 |
| 29 | 30 | |||||
Categories
- Bird
- Blog
- Conjugation of Synthetic Trisaccharide of Staphylococcus aureus Type 8 Capsular Polysaccharide Elicits Antibodies Recognizing Intact Bacterium.
- Model of vaccine efficacy against HSV-2 superinfection of HSV-1 seropositive mice demonstrates protection by antibodies mediating cellular cytotoxicity.
- My Blog
- PCR
- peptide
- Percp
- Perivascular Lymphocyte Clusters Induced by Gastric Subserous Layer Vaccination Mediate Optimal Immunity against Helicobacter through Facilitating Immune Cell Infiltration and Local Antibody Response.
- peroxidase
- phosphor
- PicoProbe
- Standard
- step
- sterile
- strip
- Target
- TEMED

